Exercises
Jammu and Kashmir Board Science Solutions for Exercises
Simple step-by-step solutions to Exercises questions of Chemical Effects of Electric Current from SCIENCE TEXTBOOK FOR CLASS 8. Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Exercises with Hints & Solutions
If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the _____ terminal of the battery.
A tester is used to check the conduction of electricity through two liquids, labelled A and B. It is found that the bulb of the tester glows brightly for liquid A while it glows very dimly for liquid B. You would conclude that
In case of a fire, before the firemen use the water hoses they shut off the main electrical supply for the area. Explain why they do this.
A child staying in a coastal region tests the drinking water and also the seawater with his tester. He finds that the compass needle deflects more in the case of seawater. Can you explain the reason?
Is it safe for the electrician to carry out electrical repairs outdoors during heavy downpour? Explain.
Paheli had heard that rainwater is as good as distilled water. So, she collected some rainwater in a clean glass tumbler and tested it using a tester. To her surprise, she found that the compass needle showed deflection. What could be the reasons?
Prepare a list of objects around you that are electroplated.
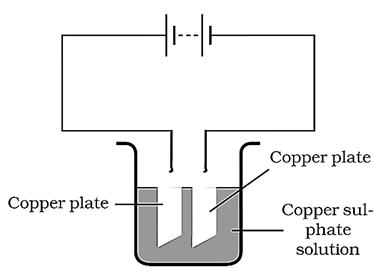
Allow the current to pass for about minutes. Now remove the electrodes from the solution and look at them carefully. Do you find any difference in any one of them? Do you find a coating over it? What colour is the coating? Note down the terminal of the battery with which this electrode is connected. The process that you saw in the above activity, is used for purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from impure rod is sought to be transferred to the thin copper plate. Which electrode should be attached to the positive terminal of the battery and why?
