K A Tsokos Solutions for Chapter: Thermal Physics, Exercise 3: Exam-style questions
K A Tsokos Physics Solutions for Exercise - K A Tsokos Solutions for Chapter: Thermal Physics, Exercise 3: Exam-style questions
Attempt the practice questions on Chapter 3: Thermal Physics, Exercise 3: Exam-style questions with hints and solutions to strengthen your understanding. Physics for the IB Diploma 6th Edition solutions are prepared by Experienced Embibe Experts.
Questions from K A Tsokos Solutions for Chapter: Thermal Physics, Exercise 3: Exam-style questions with Hints & Solutions
Define what is meant by specific heat capacity of a substance.
Consider two metals that have different specific heat capacities. The energies required to increase the temperature of 1 mole of aluminium and 1 mole of copper by the same amount are about the same. Yet the specific heat capacities of the two metals are very different. Suggest a reason for this.
Describe what is meant by the internal energy of a substance.
A student claims that the Kelvin temperature of a body is a measure of its internal energy. Explain why this statement is not correct by reference to a solid melting.
In an experiment, a heater of power is used to warm of a liquid in an uninsulated container. The graph shows the variation of the temperature of the liquid with time.
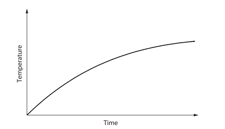
The liquid never reaches its boiling point. Suggest why the temperature of the liquid approaches a constant value.
For one vessel if Information is as given below. Then What is the Pressure of the given vessel? (in Pa)
if the pressure of the vessel is decrease from 250 kPa to 230 kPa in course of 8 hours and the volume and the temperature remain constant. What is the average rate of the molecule loss? (in molecules/sec)
(at p = 250 kPa)
For molar mass of air be And the mole reduce from 1.653 moles to 1.52 moles. What is the total mass of the air? (in gram)
