K L Chugh Solutions for Chapter: Chemical Kinetics, Exercise 9: REVIEW EXERCISES
K L Chugh Chemistry Solutions for Exercise - K L Chugh Solutions for Chapter: Chemical Kinetics, Exercise 9: REVIEW EXERCISES
Attempt the practice questions on Chapter 4: Chemical Kinetics, Exercise 9: REVIEW EXERCISES with hints and solutions to strengthen your understanding. NEW SYSTEMATIC MODERN CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from K L Chugh Solutions for Chapter: Chemical Kinetics, Exercise 9: REVIEW EXERCISES with Hints & Solutions
Consider the Arrhenius equation given below and mark the correct option.
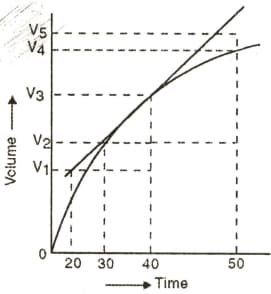
A graph of the volume of hydrogen released vs time for the reaction between zinc and dilute is given in the figure below. On the basis of this, mark the correct option.
What is the effect of temperature on the rate of reaction? Explain giving reasons.
Define threshold energy and activation energy. How are these related to each other?
What is Arrhenius equation? Discuss its importance.
What would you expect : an exothermic reaction to go fast or slow at a higher temperature and why? Do you carry out such reactions commercially at higher or lower temperature? Give reasons for your answer.
Define 'order' and 'molecularity' of a chemical reaction. Are they same always?
What is meant by the term 'catalyst' in a chemical reaction? Mention the characteristic features of catalyst.
