Kumar Mittal Solutions for Exercise 1: QUESTIONS
Kumar Mittal Physics Solutions for Exercise - Kumar Mittal Solutions for Exercise 1: QUESTIONS
Attempt the free practice questions from Exercise 1: QUESTIONS with hints and solutions to strengthen your understanding. ISC Physics Class 12, Part-2 solutions are prepared by Experienced Embibe Experts.
Questions from Kumar Mittal Solutions for Exercise 1: QUESTIONS with Hints & Solutions
A retarding potential of is required to block the movement of electrons from the cathode of a photoelectric cell when violet light of wavelength strikes its surface. Find the threshold frequency.
A retarding potential of is required to block the movement of electrons from the cathode of a photoelectric cell when violet light of wavelength strikes its surface. Find the energy light of wavelength .
A retarding potential of is required to block the movement of electrons from the cathode of a photoelectric cell when violet light of wavelength strikes its surface. Find the maximum energy of the electron emitted from the cathode surface when light strikes it.
Find the frequency of light which ejects from a metal surface electrons which are fully stopped by a retarding potential of . The photoelectric effect starts in this metal at a frequency of .
Ultraviolet light of wavelength and , when allowed to fall on hydrogen atoms in their ground state, is found to liberate electrons with kinetic energies and respectively. Find the value of Planck's cosntant.
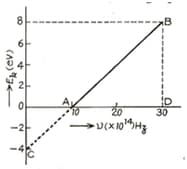
In an experiment of photoelectric effect, the graph of maximum kinetic energy of the emitted photoelectrons versus the frequency of the incident light is a straight line shown in the figure. Calculate threshold frequency.
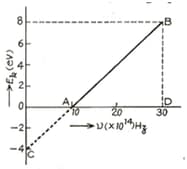
In an experiment of photoelectric effect, the graph of maximum kinetic energy of the emitted photoelectrons versus the frequency of the incident light is a straight line shown in the figure. Calculate work function of cathode-metal in .
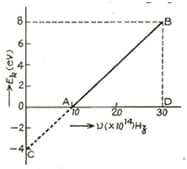
In an experiment of photoelectric effect, the graph of maximum kinetic energy of the emitted photoelectrons versus the frequency of the incident light is a straight line shown in the figure. Calculate stopping potential for the electrons emitted by light of frequency .