Lakhmir Singh and Manjit Kaur Solutions for Chapter: Periodic Classification Of Elements, Exercise 2: Long Answer Type Answer
Lakhmir Singh Chemistry Solutions for Exercise - Lakhmir Singh and Manjit Kaur Solutions for Chapter: Periodic Classification Of Elements, Exercise 2: Long Answer Type Answer
Attempt the free practice questions on Chapter 5: Periodic Classification Of Elements, Exercise 2: Long Answer Type Answer with hints and solutions to strengthen your understanding. Science for Tenth Class Part - 2 Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Lakhmir Singh and Manjit Kaur Solutions for Chapter: Periodic Classification Of Elements, Exercise 2: Long Answer Type Answer with Hints & Solutions
The following diagram shows a part of the periodic table containing first three periods in which five elements have been represented by the letters and (which are not their chemical symbols):
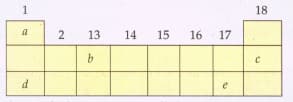
Select the letter which represents an alkali metal.
Atoms of eight elements and have the same number of electron shells but different number of electrons in their outermost shells. It was found that elements and combine to form an ionic compound. This ionic compound is added in a small amount to almost all vegetables and dishes during cooking. Oxides of elements and are basic in nature while those of elements and are acidic. The oxide of element is, however, almost neutral. Based on the above information, answer the following question:
Which two of these elements could definitely be metals?
Atoms of eight elements and have the same number of electron shells but different number of electrons in their outermost shells. It was found that elements and combine to form an ionic compound. This ionic compound is added in a small amount to almost all vegetables and dishes during cooking. Oxides of elements and are basic in nature while those of elements and are acidic. The oxide of element is, however, almost neutral. Based on the above information, answer the following question:
Which one of the eight elements is most likely to be found in gaseous state at room temperature?
Atoms of eight elements and have the same number of electron shells but different number of electrons in their outermost shells. It was found that elements and combine to form an ionic compound. This ionic compound is added in a small amount to almost all vegetables and dishes during cooking. Oxides of elements and are basic in nature while those of elements and are acidic. The oxide of element is, however, almost neutral. Based on the above information, answer the following question:
If the number of electrons in the outermost shell of elements and be and respectively, write the formula of the compound formed by the combination of and .
Write the names and symbols of two very reactive metals belonging to group of the periodic table. Explain by drawing electronic structure, how either one of the two metals reacts with a halogen. With which name is the bond formed between these elements known and what is the class of the compound so formed known? State any four physical properties of such compounds.
The non-metal is an important constituent of our food and most of the fuels around us. forms two oxides and . The oxide is poisonous whereas oxide causes global warming.
(a) Identify and .
(b) To which group of periodic table does belong?
(c) Name another element which is placed in the same group as .
A non-metal which is the largest constituent of air combines with hydrogen when heated in the presence of iron as catalyst to form a gas Y. When gas Y is treated with sulphuric acid, it forms a compound Z which is used as a chemical fertiliser.
What are X, Y and Z?
A non-metal which is the largest constituent of air combines with hydrogen when heated in the presence of iron as catalyst to form a gas . When gas is treated with sulphuric acid, it forms a compound which is used as a chemical fertiliser.
To which group of periodic table does belong?
