Lawrie Ryan and Roger Norris Solutions for Chapter: Benzene and its Compounds, Exercise 3: Question
Lawrie Ryan Chemistry Solutions for Exercise - Lawrie Ryan and Roger Norris Solutions for Chapter: Benzene and its Compounds, Exercise 3: Question
Attempt the practice questions on Chapter 25: Benzene and its Compounds, Exercise 3: Question with hints and solutions to strengthen your understanding. Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years) solutions are prepared by Experienced Embibe Experts.
Questions from Lawrie Ryan and Roger Norris Solutions for Chapter: Benzene and its Compounds, Exercise 3: Question with Hints & Solutions
Give the number of electrons involved in the bonding system in a benzene molecule.
State the type of atomic orbital that the electrons involved in the bonding system in a benzene molecule.
Explain what we mean by the term delocalised electrons in benzene.
Compare the bonding in benzene with the bonding in hex--ene.
Draw the displayed or skeletal formula of -tribromobenzene.
Draw the displayed or skeletal formula of -dichloro--nitrobenzene.
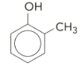
Name the molecule below:
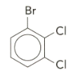
Name the molecule below: