Lawrie Ryan and Roger Norris Solutions for Chapter: Hydrocarbons, Exercise 11: Question
Lawrie Ryan Chemistry Solutions for Exercise - Lawrie Ryan and Roger Norris Solutions for Chapter: Hydrocarbons, Exercise 11: Question
Attempt the free practice questions on Chapter 15: Hydrocarbons, Exercise 11: Question with hints and solutions to strengthen your understanding. Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years) solutions are prepared by Experienced Embibe Experts.
Questions from Lawrie Ryan and Roger Norris Solutions for Chapter: Hydrocarbons, Exercise 11: Question with Hints & Solutions
Tetrafluroethene, , is the monomer for the polymer PTFE, which is used in the non-stick coating on pans. State what PTFE stands for.
Tetrafluroethene, , is the monomer for the polymer PTFE, which is used in the non-stick coating on pans. Give the name of the type of reaction to form PTFE.
Tetrafluroethene, , is the monomer for the polymer PTFE, which is used in the non-stick coating on pans. Write a chemical equation to show the formation of PTFE from .
Tetrafluroethene, , is the monomer for the polymer PTFE, which is used in the non-stick coating on pans. Draw the structure of the repeat unit in PTFE.
Tetrafluroethene, , is the monomer for the polymer PTFE, which is used in the non-stick coating on pans. How could poly(alkene) waste be used to conserve fossil fuels?
Tetrafluroethene, , is the monomer for the polymer PTFE, which is used in the non-stick coating on pans. A waste batch of poly(ethene) pellets was burnt in an inefficient industrial incinerator. Name the toxic gas that would be released.
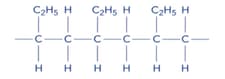
Tetrafluroethene, is the monomer for the polymer PTFE, which is used in the non-stick coating on pans. Name the monomer used to make this polymer.