Lawrie Ryan and Roger Norris Solutions for Chapter: Introduction to Organic Chemistry, Exercise 3: Question
Lawrie Ryan Chemistry Solutions for Exercise - Lawrie Ryan and Roger Norris Solutions for Chapter: Introduction to Organic Chemistry, Exercise 3: Question
Attempt the free practice questions on Chapter 14: Introduction to Organic Chemistry, Exercise 3: Question with hints and solutions to strengthen your understanding. Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years) solutions are prepared by Experienced Embibe Experts.
Questions from Lawrie Ryan and Roger Norris Solutions for Chapter: Introduction to Organic Chemistry, Exercise 3: Question with Hints & Solutions
On analysis, a hydrocarbon was found to contain of carbon and of hydrogen. Further investigation showed that the relative molecular muss of the hydrocarbon was . Deduce its molecular formula.
A compound contains the elements carbon, hydrogen and oxygen. Its empirical formula is and its relative molecular mass is . Deduce the molecular formula of the compound.
Draw the displayed formula of ethene (molecular formula ).
Draw the displayed formula of propane (molecular formula ).
Draw the skeletal formula of pentane, a straight-chain hydrocarbon with a molecular formula of .
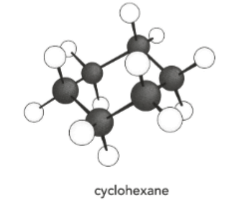
Draw the skeletal formula of the following molecule.
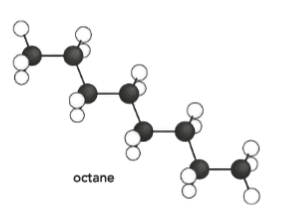
Draw the skeletal formula of the following molecule.
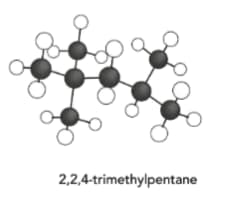
Draw the skeletal formula of the following molecule.