Lawrie Ryan and Roger Norris Solutions for Chapter: Organic Synthesis, Exercise 9: EXAM-STYLE QUESTIONS
Lawrie Ryan Chemistry Solutions for Exercise - Lawrie Ryan and Roger Norris Solutions for Chapter: Organic Synthesis, Exercise 9: EXAM-STYLE QUESTIONS
Attempt the free practice questions on Chapter 29: Organic Synthesis, Exercise 9: EXAM-STYLE QUESTIONS with hints and solutions to strengthen your understanding. Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years) solutions are prepared by Experienced Embibe Experts.
Questions from Lawrie Ryan and Roger Norris Solutions for Chapter: Organic Synthesis, Exercise 9: EXAM-STYLE QUESTIONS with Hints & Solutions
The structure of the compound known as thalidomide can be shown as:
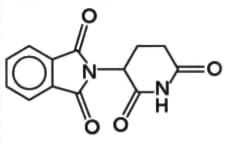
Copy the molecule and label the chiral centre on your drawing.
The structure of the compound known as thalidomide can be shown as:
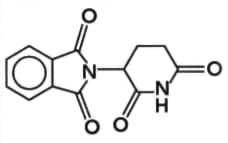
Name two functional groups found in a molecule of thalidomide.
Suggest the type of reaction that might change the molecule into an alcohol and any reagents needed.
Explain why the chirality of drugs has been such an important issue in the pharmaceutical industry, giving one benefit to patients and one benefit to pharmaceutical companies of using pure enantiomers.
The flow charts below show how poly(ethene) can be obtained by two different routes.
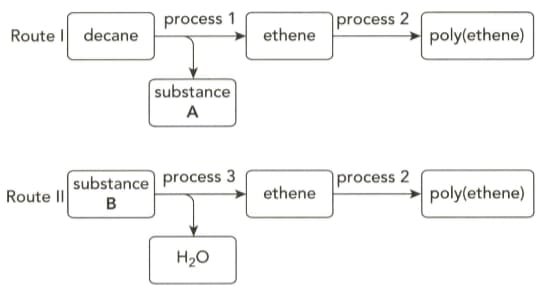
Identify substance A and give the equation for the reaction taking in process .
The flow charts below show how poly(ethene) can be obtained by two different routes.
Give the term used to describe process I.
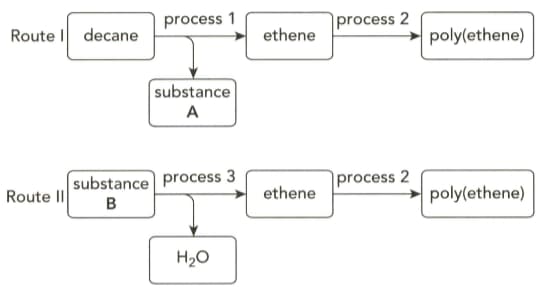
The flow charts below show how poly(ethene) can be obtained by two different routes.
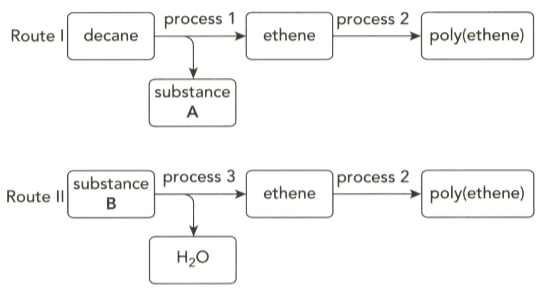
Name substance B and give the equation for the reaction taking place in process .
The flow charts below show how poly(ethene) can be obtained by two different routes.
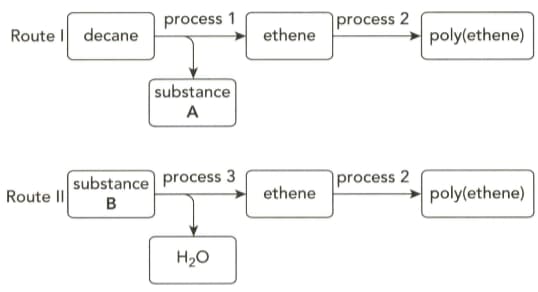
Give the term use to describe process .
