Lucy Hawkins, Fran Eardley, Stuart Lloyd and, Gemma Young Solutions for Chapter: Reactivity and Rates of Reaction, Exercise 11: Exercise 11
Lucy Hawkins Science Solutions for Exercise - Lucy Hawkins, Fran Eardley, Stuart Lloyd and, Gemma Young Solutions for Chapter: Reactivity and Rates of Reaction, Exercise 11: Exercise 11
Attempt the practice questions on Chapter 7: Reactivity and Rates of Reaction, Exercise 11: Exercise 11 with hints and solutions to strengthen your understanding. Cambridge Lower Secondary Science Stage 9: Student's Book solutions are prepared by Experienced Embibe Experts.
Questions from Lucy Hawkins, Fran Eardley, Stuart Lloyd and, Gemma Young Solutions for Chapter: Reactivity and Rates of Reaction, Exercise 11: Exercise 11 with Hints & Solutions
Describe what 'rate of reaction' means.
Look at the table below.
Explain why apparatus lost mass.
.
Look at the table below.
State the time taken for the reaction to stop.
When zinc reacts with dilute acid hydrogen is produced. State two different ways you could measure the rate of the reaction.
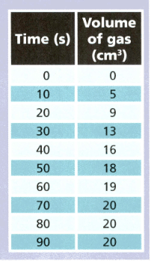
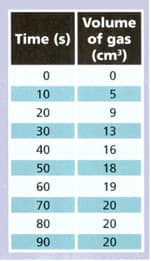
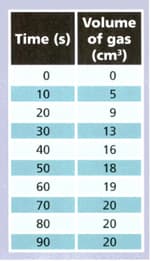
A student reacted magnesium with some hydrochloric acid and collected the gas produced. She measured the volume of gas every seconds. The table shows her results.
Use the results to answer the following questions.
During which 10 seconds was the largest volume of gas produced ?
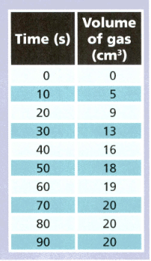
A student reacted magnesium with some hydrochloric acid and collected the gas produced. She measured the volume of gas every seconds. The table shows her results.
Use the results to answer the following questions.
What volume of gas was produced between and seconds ?
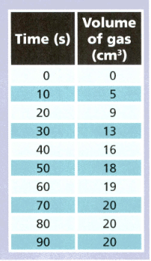
A student reacted magnesium with some hydrochloric acid and collected the gas produced. She measured the volume of gas every seconds. The table shows her results.
Use the results to answer the following questions.
When did the reaction end? Explain your answer.
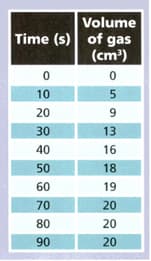
A student reacted magnesium with some hydrochloric acid and collected the gas produced. She measured the volume of gas every seconds. The table shows her results.
Use the results to answer the following questions.
At what stage was the reaction happening fastest ? Explain your answer.