Maharashtra Board Solutions for Chapter: Thermodynamics, Exercise 10: Exercises
Maharashtra Board Physics Solutions for Exercise - Maharashtra Board Solutions for Chapter: Thermodynamics, Exercise 10: Exercises
Attempt the practice questions on Chapter 4: Thermodynamics, Exercise 10: Exercises with hints and solutions to strengthen your understanding. Physics Standard 12 solutions are prepared by Experienced Embibe Experts.
Questions from Maharashtra Board Solutions for Chapter: Thermodynamics, Exercise 10: Exercises with Hints & Solutions
A system releases of heat while of work is done on the system. Calculate the change in internal energy.
Efficiency of a Carnot cycle is . If temperature of the hot reservoir is , calculate the temperature of the cold reservoir.
A Carnot refrigerator operates between and . Calculate its coefficient of performance.
An ideal gas is taken through an isothermal process. If it does of work on its environment, how much heat is added to it?
An ideal monatomic gas is adiabatically compressed so that its final temperature is twice its initial temperature. What is the ratio of the final pressure to its initial pressure?
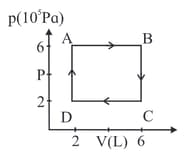
A hypothetical thermodynamic cycle is shown in the figure. Calculate the work done in 25 cycles.
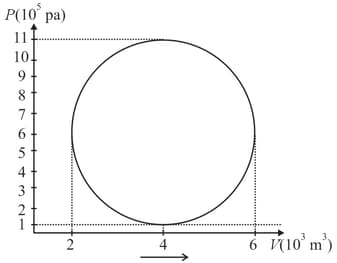
The figure shows the V-T diagram for one cycle of a hypothetical heat engine which uses the ideal gas. Draw the p-V diagram and p-T diagram of the system.
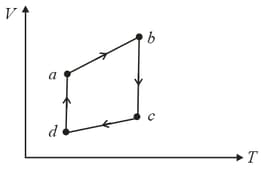
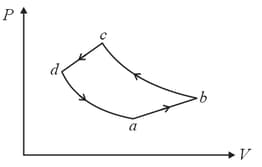
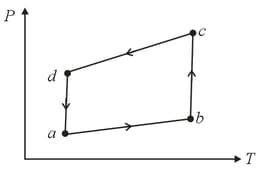
A system is taken to its final state from initial state in hypothetical paths as shown figure. Calculate the work done in each case.