Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 4: EXERCISE-4
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 4: EXERCISE-4
Attempt the free practice questions on Chapter 14: Chemical Bonding and Molecular Structure, Exercise 4: EXERCISE-4 with hints and solutions to strengthen your understanding. Beta Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 4: EXERCISE-4 with Hints & Solutions
Number of bonds and bonds in the following structure is.
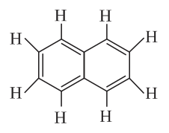
Which of the following statements are correct about
Structures of molecule of two compounds are given below:
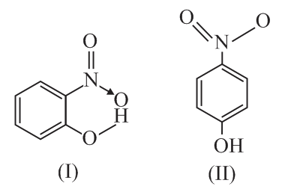
(a) Which of the two compounds will have intermolecular hydrogen bonding and which compound is expected to show intramolecular hydrogen bonding?
(b) The melting point of a compound depends on, among other things, the extent of hydrogen bonding. On this basis, explain which of the above two compounds will show higher melting point.
(c) Solubility of compounds in water depends on power to form hydrogen bonds with water. Which of the above compounds will form hydrogen bond with water easily and be more soluble in it?
In both water and dimethyl ether 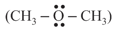 , oxygen atom is central atom, and has the same hybridisation, yet they have different bond angles. Which one has greater bond angle? Give reason.
, oxygen atom is central atom, and has the same hybridisation, yet they have different bond angles. Which one has greater bond angle? Give reason.
Give reasons for the following :
(A) Covalent bonds are directional bonds while ionic bonds are nondirectional.
(B) Water molecule has bent structure whereas carbon dioxide molecule is linear.
(C) Ethyne molecule is linear.
Elements and have and valence electrons respectively.
(A) Write the molecular formula of the compounds formed by these elements individually with hydrogen.
(B) Which of these compounds will have the highest dipole moment?
Draw the resonating structure of
(A) Ozone molecule
(B) Nitrate ion
(A) Discuss the significance/ applications of dipole moment.
(B) Represent diagrammatically the bond moments and the resultant dipole moment in and .
