NCERT Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: Short Answer Type
NCERT Chemistry Solutions for Exercise - NCERT Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: Short Answer Type
Attempt the practice questions on Chapter 4: Chemical Bonding and Molecular Structure, Exercise 1: Short Answer Type with hints and solutions to strengthen your understanding. NCERT Exemplar Chemistry - Class 11 solutions are prepared by Experienced Embibe Experts.
Questions from NCERT Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: Short Answer Type with Hints & Solutions
Structures of molecules of two compounds are given below :
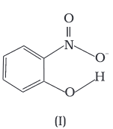
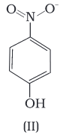
The melting point of a compound depends on, among other things, the extent of hydrogen bonding. On this basis explain which of the above two compounds will show higher melting point.
Structures of molecules of two compounds are given below :
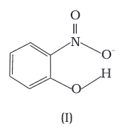
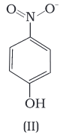
Solubility of compounds in water depends on power to form hydrogen bonds with water. Which of the above compounds will form hydrogen bond with water easily and be more soluble in it.
Give reason for the following :
Water molecule has bent structure whereas carbon dioxide molecule is linear.
Give reason for the following :
Ethyne molecule is linear.
Elements and have and valence electrons respectively.
Which of these compounds will have the highest dipole moment?
Predict the shapes of the following molecules on the basis of hybridisation.
All the bonds in carbonate ion are equal in length. Explain.
What is meant by the term average bond enthalpy? Why is there a difference in bond enthalpy of bond in ethanol and water?
