NCERT Solutions for Chapter: Structure Of The Atom, Exercise 2: Short Answer Questions
NCERT Science Solutions for Exercise - NCERT Solutions for Chapter: Structure Of The Atom, Exercise 2: Short Answer Questions
Attempt the practice questions on Chapter 4: Structure Of The Atom, Exercise 2: Short Answer Questions with hints and solutions to strengthen your understanding. NCERT Exemplar Science - Class 9 solutions are prepared by Experienced Embibe Experts.
Questions from NCERT Solutions for Chapter: Structure Of The Atom, Exercise 2: Short Answer Questions with Hints & Solutions
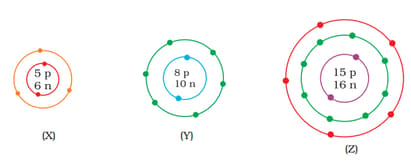
What information do you get from the figure about the atomic number, mass number and valency of atoms and ? Give your answer in a tabular form.
Match the names of the Scientists given in Column A with their contributions towards the understanding of the atomic structure as given in Column B.
| Column A | Column B |
| (a)Ernest Rutherford | (i) Indivisibility of atoms |
| (b) JJ. Thomson | (ii) Stationary orbits |
| (c) Dalton | (iii) Concept of nucleus |
| (d) Niels Bohr | (iv) Discovery of electrons |
| (e) James Chadwick | (v) Atomic number |
| (f) E. Goldstein | (vi) Neutron |
| (g) Moseley | (vii) Canal rays |
Complete the Table 4.1 on the basis of information available in the symbols given below.
(a)
(b)
(c)
| Element | ||
Complete the Table 4.1 on the basis of information available in the symbols given below.
| Element | ` |
Complete the Table 4.1 on the basis of information available in the symbols given below.
| Element | ` |
Helium atom has electrons in its valence shell but its valency is not . Explain.
Rutherford's -particle scattering experiment led to the discovery of the _____.
An element has a mass number and atomic number Write the valency of this element?
