NCERT Solutions for Chapter: Classification of Elements and Periodicity in Properties, Exercise 1: Short Answer Type
NCERT Chemistry Solutions for Exercise - NCERT Solutions for Chapter: Classification of Elements and Periodicity in Properties, Exercise 1: Short Answer Type
Attempt the practice questions on Chapter 3: Classification of Elements and Periodicity in Properties, Exercise 1: Short Answer Type with hints and solutions to strengthen your understanding. NCERT Exemplar Chemistry - Class 11 solutions are prepared by Experienced Embibe Experts.
Questions from NCERT Solutions for Chapter: Classification of Elements and Periodicity in Properties, Exercise 1: Short Answer Type with Hints & Solutions
Arrange the elements in the order of increasing first ionisation enthalpy. Give the reason for the arrangement assigned.
Arrange the elements in the order of increasing non-metallic character. Give the reason for the arrangement assigned.
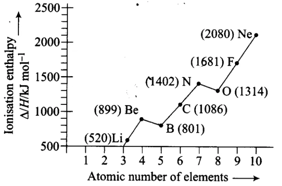
Explain the deviation In Ionisation enthalpy of some elements from the general trend by using Figure
Explain the following:
Electronegativity of elements increases on moving from left to right in the periodic table.
Explain the following:
Ionisation enthalpy decrease in a group from top to bottom?
How does the metallic and non-metallic character vary on moving from left to right in a period?
The radius of cation is less than that of atom. Give reason.
Among alkali metals which element do you expect to be least electronegative and why?