NCERT Solutions for Chapter: Hydrocarbons, Exercise 1: Short Answer Type
NCERT Chemistry Solutions for Exercise - NCERT Solutions for Chapter: Hydrocarbons, Exercise 1: Short Answer Type
Attempt the practice questions on Chapter 13: Hydrocarbons, Exercise 1: Short Answer Type with hints and solutions to strengthen your understanding. NCERT Exemplar Chemistry - Class 11 solutions are prepared by Experienced Embibe Experts.
Questions from NCERT Solutions for Chapter: Hydrocarbons, Exercise 1: Short Answer Type with Hints & Solutions
Nucleophiles and electrophiles are reaction intermediates having electron rich and electron deficient centres respectively. Hence, they tend to attack electron deficient and electron rich centres respectively. Identify the following species is electrophile or nucleophile.
The relative reactivity of , hydrogen’s towards chlorination is . Calculate the percentages of all monochlorinated products obtained from -methylbutane.
Write the structures and names of products obtained in the reactions of sodium with a mixture of -iodo--methylpropane and -iodopropane.
Write hydrocarbon radicals that can be formed as intermediates during monochlorination of -methylpropane? Which of them is more stable? Give reasons.
An alkane is obtained as the only product on subjecting a primary alkyl halide to Wurtz reaction. On monobromination this alkane yields a single isomer of tertiary bromide. Write the structure of alkane and the tertiary bromide.
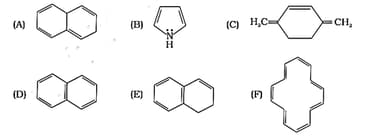
The ring systems having following characteristics are aromatic.
(i) Planar ring containing conjugated bonds.
(ii) Complete delocalisation of the -electrons in ring system i.e. each atom in the ring has unhybridised p-orbital, and
(iii) Presence of -electrons in the ring where n is an integer [Huckel rule].
Using this information classify the following compounds as aromatic/ nonaromatic.
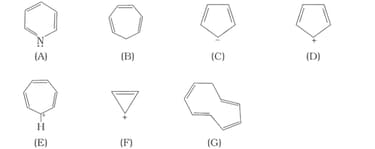
Which of the following compounds are aromatic according to Huckel’s rule?
Suggest a route to prepare ethyl hydrogen sulphate starting from ethanol
