Matching Type
Author:NCERT
NCERT Chemistry Solutions for Exercise - Matching Type
Simple step-by-step solutions to Matching Type questions of Chemical Kinetics from NCERT Exemplar Chemistry - Class 12. Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Matching Type with Hints & Solutions
MEDIUM
12th CBSE
IMPORTANT
Match the graph given in Column I with the order of reaction given in Column II. More than one item in Column I may link to the same item of Column II.
| Column I | Column II |
(i) 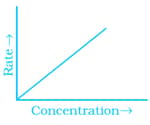 |
|
(ii) 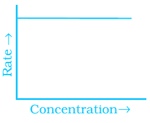 |
(a) Ist order |
(iii) 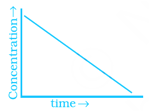 |
(b) Zero order |
(iv) 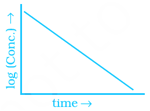 |
EASY
12th CBSE
IMPORTANT
Match the statements given in Column I and Column II
| Column I | Column II |
| (i) Catalyst alters the rate of reaction | (a) cannot be fraction or zero |
| (ii) Molecularity | (b) proper orientation is not there always |
| (iii) Second half life of first order reaction | (c) by lowering the activation energy |
| (iv) | (d) is same as the first |
| (v) Energetically favourable reactions are sometimes slow | (e) total probability is one |
| (vi) Area under the Maxwell Boltzmann curve is constant | (f) refers to the fraction of molecules with energy equal to or greater than activation energy |
MEDIUM
12th CBSE
IMPORTANT
Match the items of Column I and Column II.
| Column I | Column II |
| (i) Diamond | (a) short interval of time |
| (ii) Instantaneous rate | (b) ordinarily rate of conversion is imperceptible |
| (iii) Average rate | (c) long duration of time |
MEDIUM
12th CBSE
IMPORTANT
Match the items of Column I and Column II.
| Column I | Column II |
| (i) Mathematical expression for the rate of reaction | (a) rate constant |
| (ii) Rate of reaction for the zero-order reaction is equal to | (b) rate law |
| (iii) Units of rate constant for the zero-order reaction is same as that of | (c) order of slowest step |
| (iv) Order of a complex reaction is determined by | (d) rate of a reaction |
