Anil Ahlawat Solutions for Chapter: Acids, Bases and Salts, Exercise 1: EXERCISES
Anil Ahlawat Science Solutions for Exercise - Anil Ahlawat Solutions for Chapter: Acids, Bases and Salts, Exercise 1: EXERCISES
Attempt the free practice questions on Chapter 2: Acids, Bases and Salts, Exercise 1: EXERCISES with hints and solutions to strengthen your understanding. NSO Science Olympiad Workbook Grade 10 solutions are prepared by Experienced Embibe Experts.
Questions from Anil Ahlawat Solutions for Chapter: Acids, Bases and Salts, Exercise 1: EXERCISES with Hints & Solutions
Read the given passage and fill in the blanks by selecting an appropriate option.
Bleaching powder is a I powder. When exposed to air, it reacts with II of the air to liberate gas. It is III in cold water and the milkiness of the solution is due to the presence of unreached IV. It reacts with and liberating V gas.
| (i) | (ii) | (iii) | (iv) | ||
| (A) | Blue crystalline | Moisture | Soluble | ||
| (B) | Yellowish white | Soluble | Lime | ||
| (C) | White | Insoluble | lime | ||
| (D) | Yellow | Moisture | Soluble |
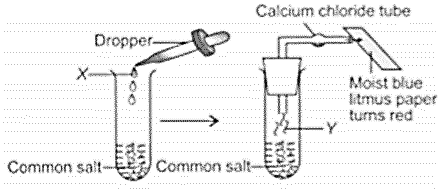
Observer the given experimental set-up carefully.
Which of the following could be the possible observation and inference drawn?
Few statements regarding the properties of bases are given below. Mark the correct statements?
(i) Sodium hydroxide and potassium hydroxide are soluble in water.
(ii) Calcium hydroxide and magnesium hydroxide are partially soluble in water.
(iii) All metallic hydroxides react with acids to form their respective metallic salts.
(iv) Metallic oxides are acidic oxides hence they react with acids to form salts.Read the given statements and mark the correct option.
Statement 1: A solution of has hydrogen ion concentration times than that of solution of .
Statement 2:Baking powder is a mixture of and mild edible acid such as . When it is heated, is produced that makes bread and cake soft and spongy. and are respectively:
A metal carbonate on reacting with acid gives a gas which when passed through a solution gives the carbonate back. Solution reacts with gas to form a compound used for decolourisation. Identify
| W | X | Y | Z | P | |
| (A) | |||||
| (B) | |||||
| (C) | |||||
| (D) |
Match column I with column II and mark the correct option from the given codes.
| Column I | Column II | ||
| (a) | (i) | Used for disinfecting water | |
| (b) | (ii) | Used in soda-acid fire extinguishers | |
| (c) | (iii) | Used for removing permanent hardness of water | |
| (d) | (iv) | Used for making toys, materials for decoration |
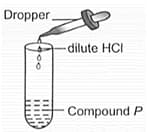
Study the given diagram carefully and identify and , respectively.