Odisha Board Solutions for Chapter: The Solid State, Exercise 1: QUESTIONS
Odisha Board Chemistry Solutions for Exercise - Odisha Board Solutions for Chapter: The Solid State, Exercise 1: QUESTIONS
Attempt the free practice questions on Chapter 1: The Solid State, Exercise 1: QUESTIONS with hints and solutions to strengthen your understanding. Bureau's Higher Secondary Chemistry Volume-2 solutions are prepared by Experienced Embibe Experts.
Questions from Odisha Board Solutions for Chapter: The Solid State, Exercise 1: QUESTIONS with Hints & Solutions
If edge of a bcc crystal of an element is , is the atomic mass and is the Avogadro number, then density of the crystal is
The unit cell with crystallographic dimensions , is
The structure of sodium chloride is
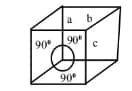
The unit cell with the structure below refers to _____ crystal system.
The arrangement ABCABC...... is referred to as
Which among the following will show anisotropy?
For an octahedral arrangement the lowest radius ratio limit is
The number of octahedral voids in a unit cell of a cubic close packed structure is
