Formative Assessment
Dr. Parul Srivastava Chemistry Solutions for Exercise - Formative Assessment
Simple step-by-step solutions to Formative Assessment questions of Chemical Reactions and Equations from PRACHI SCIENCE CHEMISTRY CLASS-X. Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Formative Assessment with Hints & Solutions
When ferrous sulphate is heated the following reaction takes place:
The above reaction is called _____of ferrous sulphate.
How the colour changes when the gases after thermal decomposition of ferrous sulphate come in contact with an acidified solution of potassium dichromate?
What is the colour of solid ferrous sulphate?
With a labelled diagram, describe an activity to find out the conditions under which iron rusts.
Solid sodium carbonate was taken in a container and water was added slowly to it. What will you observe in this reaction? Write the name and chemical formula of the product.
Write activity to show the change in the state of matter and change in temperature during a chemical reaction (change).
Write activity to show the electrolysis of water, as an example of decomposition reaction.
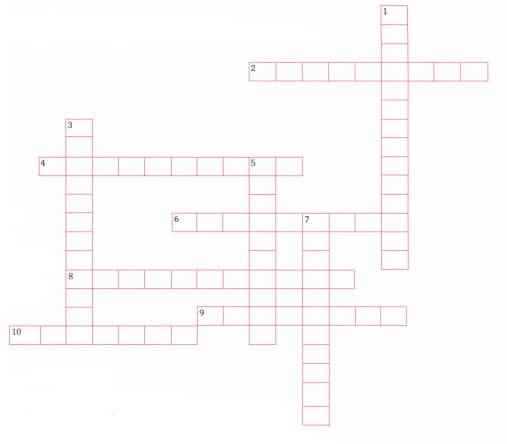
Make a crossword puzzle and solve it with the help of following clues.
ACROSS
2. An example of a decomposition reaction.
4. In a chemical reaction atoms are.
6. Another name for a combination reaction.
8. Energy is transferred from the surroundings to the reactants in this type of reaction.
9. Substances that takes oxygen.
10. An example of an exothermic reaction.
DOWN
1. An example of an endothermic reaction.
3. A chemical equation is balanced by changing or adding _____.
5. In this reaction energy is transferred from the reactant to the surroundings.
7. When a chemical reaction and its reverse reaction are occurring at the same time and at the same rate.