PRIYANKA B Solutions for Chapter: Acids, Bases and Salts, Exercise 1: Value Based Questions
PRIYANKA B Chemistry Solutions for Exercise - PRIYANKA B Solutions for Chapter: Acids, Bases and Salts, Exercise 1: Value Based Questions
Attempt the free practice questions on Chapter 2: Acids, Bases and Salts, Exercise 1: Value Based Questions with hints and solutions to strengthen your understanding. ESSENTIALS OF CHEMISTRY X solutions are prepared by Experienced Embibe Experts.
Questions from PRIYANKA B Solutions for Chapter: Acids, Bases and Salts, Exercise 1: Value Based Questions with Hints & Solutions
Quick lime is a :
The pH value of a sample of hydrochloric acid is 2. pH value of this sample when diluted by adding water will be:
If is added to distilled water, the pH of new solution will be :
When a few drops of universal indicator were added to a dilute solution of , it is observed that the colour of the solution changes from:
A student added a drop of universal indicator to 1 ml of the given solution and found that a green colour is produced. pH value of the solution will be in the range of :
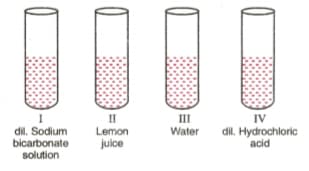
A student was provided with four samples of solutions as shown in figures (I), (Il), (Ill), and (IV). He determined pH value of each solution by using pH paper. The correct sequence of colour change of pH paper observed by the student will be:
The products of reaction between zinc and sodium hydroxide solution are :
A student was given a solution to find its pH. His teacher declared his recorded pH as wrong. Student explained to his teacher, all the steps done by him while finding pH of sample. Mark the step taken by student in which he committed mistake.
