Paul Morris Solutions for Chapter: What's in an Atom?, Exercise 15: SOME SUMMATIVE PROBLEMS TO TRY
Paul Morris Physics Solutions for Exercise - Paul Morris Solutions for Chapter: What's in an Atom?, Exercise 15: SOME SUMMATIVE PROBLEMS TO TRY
Attempt the practice questions on Chapter 11: What's in an Atom?, Exercise 15: SOME SUMMATIVE PROBLEMS TO TRY with hints and solutions to strengthen your understanding. MYP By Concept 4&5 Physics solutions are prepared by Experienced Embibe Experts.
Questions from Paul Morris Solutions for Chapter: What's in an Atom?, Exercise 15: SOME SUMMATIVE PROBLEMS TO TRY with Hints & Solutions
A factory uses two kinds of small, low radioactivity sources in the manufacture of smoke alarms. Workers are given these health and safety instructions:
Type 1 sources
- Always wear latex rubber gloves when handling
- Type 1 radioactive sources.
- Do not eat or drink when handling Type 1 radioactive sources.
- Always wear latex rubber gloves and use tongs when handling Type 2 radioactive sources.
- Do not eat or drink when handling Type 2 radioactive sources.
- Always hold Type 2 sources at least 10 cm away from the body.
State which radioactive sources most likely correspond to Type-1 and Type-2?
With reference to information in the table, outline the reason for each of the health and safety instructions.
| Name | What is it? | Electric charge | Mass(atomic mass unit) |
| alpha | 2 protons+2 neutrons | Positive | 4 |
| beta | High energy electron | Negative | 1/1837 |
| gamma | Part of the electromagnetic spectrum | None | 0 |
Carbon-14 undergoes beta decay. Write a beta decay equation for carbon-14.
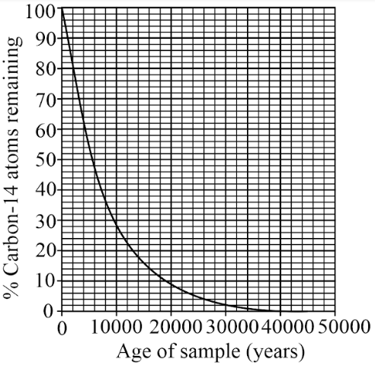
Use the graph to find the half life of carbon-14 in years.
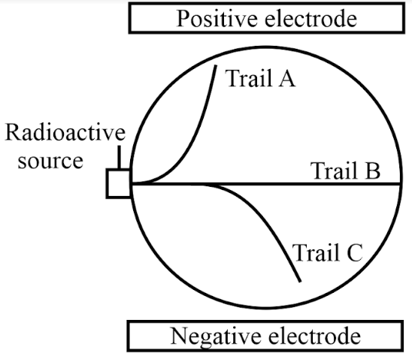
Identify and state which trail (A, B, C) was made by which type of radiation?
The scientist carrying out experiment notices that the curve of beta track is always much sharper than that of the alpha particle. Explain why this might be?
What is meant by the word isotope?
