Prof. Santosh Yadav and Prof. Anil Thomas Solutions for Chapter: Organic Reactions, Exercise 1: Exercise 1
Prof. Santosh Yadav Chemistry Solutions for Exercise - Prof. Santosh Yadav and Prof. Anil Thomas Solutions for Chapter: Organic Reactions, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 12: Organic Reactions, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. MHT-CET TRIUMPH Chemistry Multiple Choice Questions Part - 2 Based on Std. XI & XII Syllabus of MHT-CET solutions are prepared by Experienced Embibe Experts.
Questions from Prof. Santosh Yadav and Prof. Anil Thomas Solutions for Chapter: Organic Reactions, Exercise 1: Exercise 1 with Hints & Solutions
Identify and in the following reaction:
The compounds and are the structural isomers of each other with the molecular formula . Compound is a linear chain and optically inactive while is optically active. When compound is treated with aqueous solution, it gives compound whereas compound when treated with aqueous solution, it gives compound . Identify compounds and .
The compound '' undergoes Kolbe's reaction in the presence of to give the compound ''. Compound '' when treated with acetic anhydride in acidic medium, gives acetylsalicylic acid, i.e., aspirin. Identify the compounds '' and ''.
Ethyl bromide is subjected to the ammonolysis reaction to give the compound . The compound reacts with nitrous acid in cold conditions to give the compound with the liberation of the nitrogen gas. What is the molecular weight of the compound ?
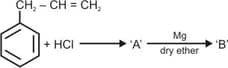
The compound in the above reaction is used as an anionic detergent in soap industry. Give the hydrophilic and hydrophobic parts of the compound .
Which one of the following polymers belongs to the class of addition polymers? Which catalyst is used to prepare its monomer from ethanol?
Nylon
Polyethene Dextron
In the following set of reactions, the alkyl halide is converted into nitrile and Grignard reagent. Both of these are used to further furnish a carboxylic acid containing one carbon atom more than the starting alkyl halide.
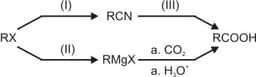
What are the reagents in these reactions?
The compound is widely used in cosmetics as a nail-polish remover. This compound forms compound , on treatment with hydroxylamine. The compound is easily reduced by sodium and ethanol to give isopropylamine. Identify the compound .
