Ramendra C Mukerjee Solutions for Chapter: Miscellaneous Problems for Revision, Exercise 1: Exercise 1
Ramendra C Mukerjee Chemistry Solutions for Exercise - Ramendra C Mukerjee Solutions for Chapter: Miscellaneous Problems for Revision, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 21: Miscellaneous Problems for Revision, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Modern Approach to Chemical Calculations solutions are prepared by Experienced Embibe Experts.
Questions from Ramendra C Mukerjee Solutions for Chapter: Miscellaneous Problems for Revision, Exercise 1: Exercise 1 with Hints & Solutions
A sample of an unknown monoprotic organic acid is dissolved in water and titrated with a sodium hydroxide solution. After the addition of of base, a of $4.92$ is recorded. The equivalence point is reached when a total of of is added.
What is the molar mass of the organic acid?
A sample of an unknown monoprotic organic acid is dissolved in water and titrated with a sodium hydroxide solution. After the addition of of base, a of is recorded. The equivalence point is reached when a total of of is added.
What is the value for the acid? The value could have been determined very easily if a measurement had been made after the addition of of . Why?
In the following equilibrium
When mole of each are taken, the temperature is kept at . The total pressure was found to be bar. Given: and , find of the reaction
In the following equilibrium
When mole of each are taken, the temperature is kept at . The total pressure was found to be bar. Given: and , the direction of the reaction in which the equilibrium shifts.
If is titrated with , calculate the at the end point. and .
(a) Calculate the velocity of electrons in the first Bohr orbit of hydrogen atom.
(b) Find the de Broglie wavelength of the electron in the first orbit.
(c) Find the orbital angular momentum of the orbital in terms of units.
For the reaction
Given:
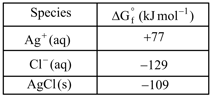
calculate
represent the reaction as cell calculate & find for .
of metallic was added to of saturated solution of . Calculate .
Given:
Also find how many moles of Ag will be formed.
