Richard Harwood and Ian Lodge Solutions for Chapter: Quantitative Chemistry, Exercise 2: Exercise 6.2
Richard Harwood Chemistry Solutions for Exercise - Richard Harwood and Ian Lodge Solutions for Chapter: Quantitative Chemistry, Exercise 2: Exercise 6.2
Attempt the practice questions on Chapter 6: Quantitative Chemistry, Exercise 2: Exercise 6.2 with hints and solutions to strengthen your understanding. Cambridge IGCSE Chemistry Workbook 4th Edition solutions are prepared by Experienced Embibe Experts.
Questions from Richard Harwood and Ian Lodge Solutions for Chapter: Quantitative Chemistry, Exercise 2: Exercise 6.2 with Hints & Solutions
Zinc metal is extracted from its oxide. In the industrial extraction process, 5 tonnes of zinc oxide are needed to produce 4 tonnes of zinc. Calculate the mass of zinc, in tonnes, that is produced from 20 tonnes of zinc oxide.
Nitrogen and hydrogen react together to form ammonia.
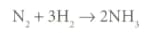
When the reaction is complete, 14 tonnes of nitrogen are converted into 17 tonnes of ammonia. How much nitrogen will be needed to produce 34 tonnes of ammonia?
The sugar lactose,, is sometimes used in place of charcoal in fireworks. State the total number of atoms present in a molecule of lactose.
A molecule of compound Y contains the following atoms bonded covalently together:
• 2 atoms of carbon (C)
• 2 atoms of oxygen (O)
• 4 atoms of hydrogen (H).
What is the formula of a molecule of Y?
