RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Cambridge IGCSE Exam Questions from Paper 3, Exercise 2: CAMBRIDGE IGCSE EXAM QUESTIONS from Paper 3
RoseMarie Gallagher Chemistry Solutions for Exercise - RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Cambridge IGCSE Exam Questions from Paper 3, Exercise 2: CAMBRIDGE IGCSE EXAM QUESTIONS from Paper 3
Attempt the free practice questions on Chapter 21: Cambridge IGCSE Exam Questions from Paper 3, Exercise 2: CAMBRIDGE IGCSE EXAM QUESTIONS from Paper 3 with hints and solutions to strengthen your understanding. Complete Chemistry for Cambridge IGCSE® Second Edition solutions are prepared by Experienced Embibe Experts.
Questions from RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Cambridge IGCSE Exam Questions from Paper 3, Exercise 2: CAMBRIDGE IGCSE EXAM QUESTIONS from Paper 3 with Hints & Solutions
The results of experiments on electrolysis using inert electrodes are given in the table.
| electrolyte | change at the negative electrode | change at the positive electrode | change to electrolyte |
| molten lead(II) bromide | lead formed | bromine formed | Used |
| _________ | potassium formed | iodine formed | used up |
| dilute aqueous sodium chloride | _______ | ________ | ________ |
| aqueous copper sulphate | _________ | ________ | _________ |
| __________ | hydrogen formed | bromine formed | potassium hydroxide formed |
Complete the table; the first line has been completed as an example.
Complete the labelling of the diagram.
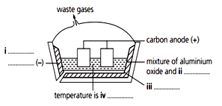
When nitric acid is added to water the following reaction occurs.
Give the name and the formula of the particle transferred from nitric acid to water.
This question is about the following oxides. Aluminium oxide , Calcium oxide , Carbon dioxide, Carbon monoxide , `Magnesium oxide , Sulphur dioxide
(i) Which will react with hydrochloric acid but not with aqueous sodium hydroxide?
This question is about the following oxides. Aluminium oxide , Calcium oxide , Carbon dioxide, Carbon monoxide , `Magnesium oxide , Sulphur dioxide
Which will react with aqueous sodium hydroxide but not with hydrochloric acid?
gt;This question is about the following oxides. Aluminium oxide , Calcium oxide , Carbon dioxide, Carbon monoxide , `Magnesium oxide , Sulphur dioxide
(iii) Which will react both with hydrochloric acid and aqueous sodium hydroxide?
>This question is about the following oxides. Aluminium oxide , Calcium oxide , Carbon dioxide, Carbon monoxide , `Magnesium oxide , Sulphur dioxide
(iv) Which will react neither with hydrochloric acid nor with aqueous sodium hydroxide?
The table below gives the heats of combustion of the first three alcohols.
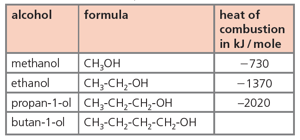
Determine the heat of combustion of butan- 1-ol by plotting the heats of combustion of the first three alcohols against the number of carbon atoms per molecule. Label your graph as on the right.
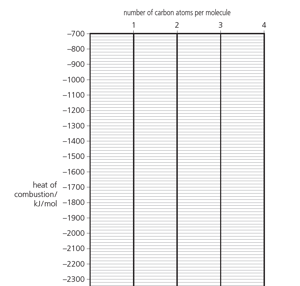
What is the heat of combustion of butan-1-ol in kJ / mol?
