RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Reacting Masses, and Chemical Equations, Exercise 5: Checkup on Chapter 5
RoseMarie Gallagher Chemistry Solutions for Exercise - RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Reacting Masses, and Chemical Equations, Exercise 5: Checkup on Chapter 5
Attempt the free practice questions on Chapter 5: Reacting Masses, and Chemical Equations, Exercise 5: Checkup on Chapter 5 with hints and solutions to strengthen your understanding. Complete Chemistry for Cambridge IGCSE® Second Edition solutions are prepared by Experienced Embibe Experts.
Questions from RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Reacting Masses, and Chemical Equations, Exercise 5: Checkup on Chapter 5 with Hints & Solutions
This shows the structure of a molecular compound. Name the compound.
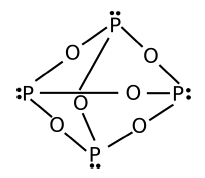
This shows the structure of a molecular compound. What is the simplest formula of it?
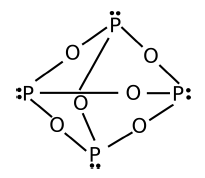
Calculate the relative formula mass for the ionic compound Potassium carbonate, .
Calculate the relative formula mass for the ionic compound hydrated Iron sulphate, .
Aluminium is extracted from the ore Bauxite, which is impure Aluminium oxide. of the ore was found to have this composition:
Aluminium oxide , Iron oxide and sand
What percentage of this ore has impurities?
Aluminium is extracted from the ore Bauxite, which is impure Aluminium oxide. of the ore was found to have this composition:
Aluminium oxide , Iron oxide and sand .
of ore gives of Aluminium. How much aluminium will be obtained from of the ore?
Aluminium is extracted from the ore Bauxite, which is impure Aluminium oxide. of the ore was found to have this composition:
Aluminium oxide , Iron oxide and sand
of ore gives of Aluminium. What mass of sand is in this ?
Aluminium is extracted from the ore Bauxite, which is impure Aluminium oxide. of the ore was found to have this composition:
Aluminium oxide , Iron oxide and sand .
What will be the percentage of Aluminium oxide in the ore, if all the Iron oxide is removed, leaving only the Aluminium oxide and sand?
