RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Separating Substances, Exercise 1: Q
RoseMarie Gallagher Chemistry Solutions for Exercise - RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Separating Substances, Exercise 1: Q
Attempt the free practice questions on Chapter 2: Separating Substances, Exercise 1: Q with hints and solutions to strengthen your understanding. Complete Chemistry for Cambridge IGCSE® Second Edition solutions are prepared by Experienced Embibe Experts.
Questions from RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Separating Substances, Exercise 1: Q with Hints & Solutions
Look at the table and answer:
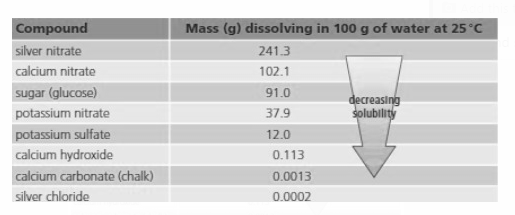
Silver nitrate gives a colourless solution. What will you see if you add g of it to g of water at ?
Look at the table and answer:
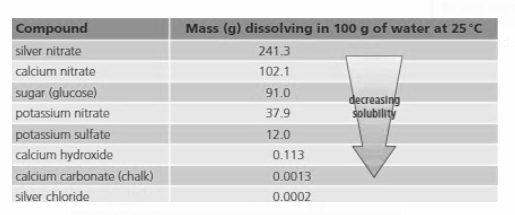
What will you see if you heat up the saturated solution of silver nitrate?
Look at the table and answer:
| Soluble | Insoluble | |
| All sodium, potassium and ammonium salts | ||
| All nitrates | ||
| Chlorides | Except | Silver and lead chloride |
| Sulphates | Except | Calcium, barium and lead sulphate but all other carbonates are insoluble |
| Sodium, potassium and ammonium carbonates |
Name two metals that have no insoluble salts.
Look at the table and answer:
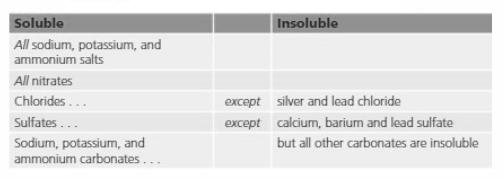
Name one other group of salts that are always soluble.
Give three examples of solids you dissolve in water, at home.
Give three examples of insoluble solids you use at home.
Name two solvents other than water that is used in the home. What are they used for?
Many gases dissolved in the water. Try to give some examples.
