RoseMarie Gallagher and Paul Ingram Solutions for Chapter: States of Matter, Exercise 4: Q
RoseMarie Gallagher Chemistry Solutions for Exercise - RoseMarie Gallagher and Paul Ingram Solutions for Chapter: States of Matter, Exercise 4: Q
Attempt the practice questions on Chapter 1: States of Matter, Exercise 4: Q with hints and solutions to strengthen your understanding. Complete Chemistry for Cambridge IGCSE® Second Edition solutions are prepared by Experienced Embibe Experts.
Questions from RoseMarie Gallagher and Paul Ingram Solutions for Chapter: States of Matter, Exercise 4: Q with Hints & Solutions
What causes the pressure in a gas?
Why does a balloon burst if you keep on blowing?
A gas is in a sealed container. How do you think the pressure will change if the container is cooled? Explain your answer.
Why does the scent of perfume spread?
Why does the scent of perfume wear off faster in warm weather than in cold?
Of all gases, hydrogen diffuses fastest at any given temperature. What can you tell from this?
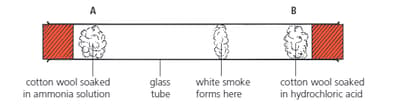
Look at the glass tube below. Suppose it was warmed a little in an oven, before the experiment. Do you think that would change the result? If so, how?
The particles in hydrogen chloride gas are twice as heavy as those in ammonia gas.
• Cotton wool soaked in ammonia solution is put into one end of a long tube (at A below). It gives off ammonia gas.
• At the same time, cotton wool soaked in hydrochloric acid is put into the other end of the tube (at B). It gives off hydrogen chloride gas.
• The gases diffuse along the tube. White smoke forms where they meet: