RoseMarie Gallagher and Paul Ingram Solutions for Chapter: The Behaviour of Metals, Exercise 5: Q
RoseMarie Gallagher Chemistry Solutions for Exercise - RoseMarie Gallagher and Paul Ingram Solutions for Chapter: The Behaviour of Metals, Exercise 5: Q
Attempt the free practice questions on Chapter 13: The Behaviour of Metals, Exercise 5: Q with hints and solutions to strengthen your understanding. Complete Chemistry for Cambridge IGCSE® Second Edition solutions are prepared by Experienced Embibe Experts.
Questions from RoseMarie Gallagher and Paul Ingram Solutions for Chapter: The Behaviour of Metals, Exercise 5: Q with Hints & Solutions
A copper rod and an iron rod stand in an electrolyte. If you connect a bulb between them, it will light dimply. Which acts as the positive electrode: copper or iron?
A copper rod and an iron rod stand in an electrolyte. If you connect a bulb between them, it will light dimply. Suggest two metals you could use to get a brighter light.
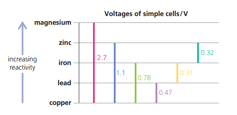
From the chart, see if you can work out the voltage for a cell that uses magnesium and zinc.
Steel for cars is galvanised. What does that mean?
Explain how galvanisation protects the steel.
Aluminium is more reactive than iron. But unlike iron, we do need to protect it from corrosion. Why not?
Write a word equation for the thermite reaction.
See if you can give two reasons why the aluminium is powdered, for thermite reaction.
