RoseMarie Gallagher and Paul Ingram Solutions for Chapter: The Periodic Table, Exercise 4: Q
RoseMarie Gallagher Chemistry Solutions for Exercise - RoseMarie Gallagher and Paul Ingram Solutions for Chapter: The Periodic Table, Exercise 4: Q
Attempt the free practice questions on Chapter 12: The Periodic Table, Exercise 4: Q with hints and solutions to strengthen your understanding. Complete Chemistry for Cambridge IGCSE® Second Edition solutions are prepared by Experienced Embibe Experts.
Questions from RoseMarie Gallagher and Paul Ingram Solutions for Chapter: The Periodic Table, Exercise 4: Q with Hints & Solutions
One measurement in the table shown below does not fit the trend. Identify the measurement and give the reason for the same. 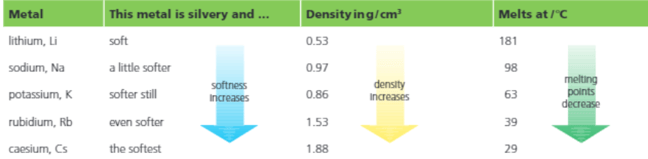
Which compound is formed when potassium reacts with chlorine?
What is the colour of the compound formed when potassium reacts with chlorine?
What will you see when you dissolve potassium chloride in water?
Potassium chloride formed on reacting potassium with chlorine is mixed with water. Discuss whether the solution conducts electricity or not.
Which holds its outer electron more strongly: a lithium atom or a sodium atom? Explain why you think so?
Rubidium is below potassium, in Group . Predict how it will react with water.
Rubidium is below potassium, in Group . Predict how it will react with chlorine.
