S C Kheterpal, S N Dhawan and, P N Kapil Solutions for Chapter: Equilibrium in Physical and Chemical Processes, Exercise 24: Competition Focus
S C Kheterpal Chemistry Solutions for Exercise - S C Kheterpal, S N Dhawan and, P N Kapil Solutions for Chapter: Equilibrium in Physical and Chemical Processes, Exercise 24: Competition Focus
Attempt the practice questions on Chapter 8: Equilibrium in Physical and Chemical Processes, Exercise 24: Competition Focus with hints and solutions to strengthen your understanding. Pradeep's Chemistry Vol 1 solutions are prepared by Experienced Embibe Experts.
Questions from S C Kheterpal, S N Dhawan and, P N Kapil Solutions for Chapter: Equilibrium in Physical and Chemical Processes, Exercise 24: Competition Focus with Hints & Solutions
Which of the following lines correctly show the temperature dependence of equilibrium constant , for an exothermic reaction?
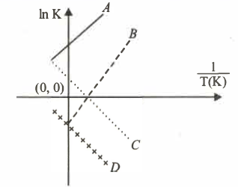
Which of the following are reversible reactions?
Which of the following statements are wrong?
The equilibrium is attained at in a closed container and inert gas helium is introduced. Which of the following statements are correct?
For the reaction , the forward reaction at constant temperature is favoured by
The equilibrium in aqueous medium at shifts towards the left in the presence of
The thermal dissociation equilibrium of is studied under different conditions.
For this equilibrium, the correct statement (s) is (are)
For the following question, enter the correct numerical value, (in decimal-notation, truncated/rounded-off to the second decimal place, e.g., ).
The approach of the following equilibrium was observed kinetically from both directions :
At , it was found that
What will be the value of the equilibrium constant when fourth ion is complexed, i.e., for the step involving backward reaction?
