S C Kheterpal, S N Dhawan and, P N Kapil Solutions for Chapter: Thermodynamics, Exercise 18: Competition Focus
S C Kheterpal Chemistry Solutions for Exercise - S C Kheterpal, S N Dhawan and, P N Kapil Solutions for Chapter: Thermodynamics, Exercise 18: Competition Focus
Attempt the practice questions on Chapter 7: Thermodynamics, Exercise 18: Competition Focus with hints and solutions to strengthen your understanding. Pradeep's Chemistry Vol 1 solutions are prepared by Experienced Embibe Experts.
Questions from S C Kheterpal, S N Dhawan and, P N Kapil Solutions for Chapter: Thermodynamics, Exercise 18: Competition Focus with Hints & Solutions
The number of properties which are state functions among the following is
Pressure, Volume, Temperature, Heat, Work, Entropy, Enthalpy, Free energy, Internal energy.
A gas expands against a constant external pressure so that the work done is . The work done in litre atmosphere is
The number of moles of an ideal gas that should be taken in a closed vessel of capacity at a temperature of so that the pressure exerted by the gas on the walls of the container is atmosphere is
If for a particular reaction, the difference in the heat evolved when the reaction is carried out at constant pressure and that at constant volume at is nearly , then the difference in the number of moles of gaseous reactants and products is
The ratio of value of a triatomic gas to the value of a monatomic gas is
The enthalpy of neutralisation of will be how many times the enthalpy of neutralisation of
In a constant volume calorimeter, of a gas with molecular weight was burnt in excess oxygen at . The temperature of the calorimeter was found to increase from due to the combustion process. Given that the heat capacity of the calorimeter is , the numerical value for the enthalpy of combustion of the gas in is
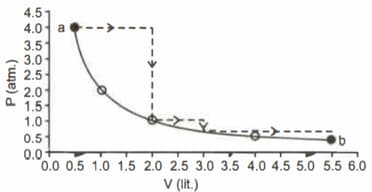
One mole of an ideal gas is taken from along two paths denoted by the solid and the dashed lines as shown in the graph below. If the work done along the solid line path is and that along the dotted line path is , then the integer closest to the ratio is