Saleha Pervez and Shikha Goel Solutions for Chapter: Periodic Properties and Their Variations, Exercise 6: ARCHIVES* (Last 6 Years)
Saleha Pervez Chemistry Solutions for Exercise - Saleha Pervez and Shikha Goel Solutions for Chapter: Periodic Properties and Their Variations, Exercise 6: ARCHIVES* (Last 6 Years)
Attempt the practice questions on Chapter 1: Periodic Properties and Their Variations, Exercise 6: ARCHIVES* (Last 6 Years) with hints and solutions to strengthen your understanding. All in One - ICSE Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Saleha Pervez and Shikha Goel Solutions for Chapter: Periodic Properties and Their Variations, Exercise 6: ARCHIVES* (Last 6 Years) with Hints & Solutions
Arrange the following as per the instruction given in the bracket.
(Increasing order of the number of electron shells).
Arrange the following as per the instruction given in the bracket.
(Increasing ionisation energy).
Arrange the following as per the instruction given in the bracket.
(Increasing electronegativity).
Arrange the following as per the instruction given in the bracket.
(Increasing atomic size).
Match the atomic number and with each of the following:
(i) A solid non-metal belonging to the third period.
(ii) A metal of valency .
(iii) A gaseous element with valency .
(iv) An element belonging to group .
(v) A rare gas.
Identify the term/substance in the following
The tendency of an atom to attract electrons towards itself when combined in a compound.
Rewrite the following sentence by using the correct symbol (greater than) or (less than) in the blank given.
The ionisation potential of potassium is less than _____ that of sodium.
Use the letters only written in the periodic table given below to answer the questions that follow
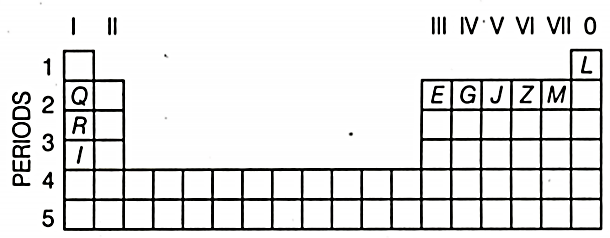
(i) State the number of valence electrons in atom .
(ii) Which element forms ions with a single negative charge?
(iii) Out of and which one is more reactive?
(iv) Which element has its electrons arranged in four shells?
