Chapter Practice
Saleha Pervez Chemistry Solutions for Exercise - Chapter Practice
Simple step-by-step solutions to Chapter Practice questions of Chemical Kinetics from All In One ISC Chemistry Class 12. Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Chapter Practice with Hints & Solutions
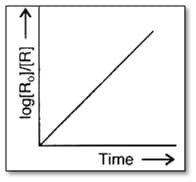
Answer the following question on the basis of the curve given below for a zero order reaction :
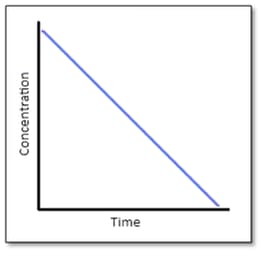
Find the relation between slope of this line and rate constant.
Answer the following question on the basis of the curve given below for a zero order reaction :
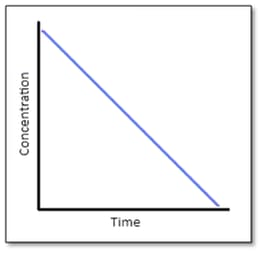
If the slope is , then, calculate the rate constant.
Consider the reaction, . The change in concentration with time is shown in the graph. Predict the order of the reaction.
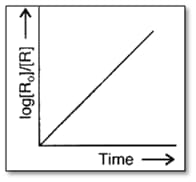
Consider the reaction, . The change in concentration with time is shown in the graph. Derive the expression for the time which is
required to complete the reaction.
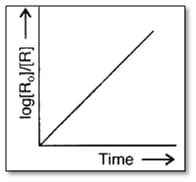
Consider the reaction, . The change in concentration with time is shown in the graph. Predict the order of the reaction.
changes to by successive radioactive decay. A sample of uranium was analyzed and found to contain of and of had accumulated due to decay of, find out the age of ore. (Half-life of years).
For the hydrolysis of methyl acetate in the aqueous solution, following results were obtained :
Show that it follows pseudo first order reaction, as the concentration of water remains constant.
For the hydrolysis of methyl acetate in the aqueous solution, following results were obtained :
Calculate the average rate of reaction between the time interval to .
