Valency
Important Questions on Valency
Match the elements in column A with the valency exhibited by the element given in column B.
| Column A | Column B |
| (A) Neon (B) Oxygen (C) Potassium (D) Boron (E) Silicon |
(a) (b) (c) (d) (e) |
What would be the valency of an element that is chemically inactive?
Aarya studied the electronic configurations of some of the elements. He made a graph for the atomic number of the elements and their outer shell electrons. He plotted two different lines on his graph on the basis of the difference in their outer shell electrons. Experimentally he took one element from each of the groups and found that the element from series two shows an exothermic reaction with water while the element from series one does not react at all.
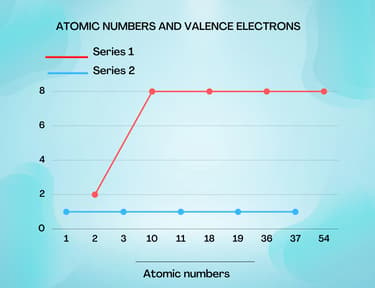
- Series one is not a straight line while series two has. Could you explain what difference it explains?
- What groups of elements do these series represent?
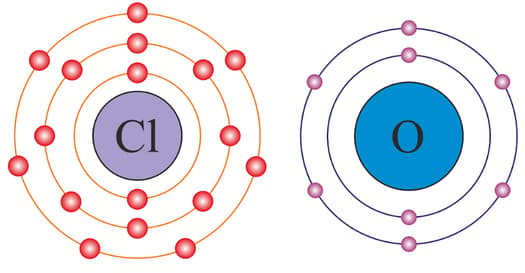
Two elements, and , belong to groups and respectively in the same period of the Periodic Table. One day, both and elements went shopping. Element purchased a chlorine atom and element purchased an oxygen atom. The next day, they again went to the same shop and bought chlorine and oxygen. On the third day, when they went shopping, the shop owner asked, "Why are you purchasing only oxygen and chlorine?" Is there any reason behind it? Then and elements said, "Yes, there is beautiful chemistry behind this. We are choosing elements based upon our combining capacity. That is why we are purchasing oxygen and chlorine atoms because these elements easily accept our electrons." Now can you compare these elements with respect to their valencies?
A chemical compound has the following Lewis notation:
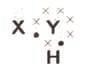
What is the valency of element ?
A chemical compound has the following Lewis notation:
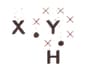
How many valence electrons does element have?
If the valency of Carbon is and that of Hydrogen is , then the molecular formula of methane is _____.
(A) (B) (C)
Enter your correct answer as A, B or C.
Use the following molecular formulae to determine the valencies of .
Molecular formulae of
'A' has protons, electrons and neutrons. 'B' has protons, electrons and neutrons. Formula between A and B is
The atomic numbers of and are and respectively. What will be the number of electrons in and ?
Which element will be more reactive- the element whose atomic number is 18 or the one whose atomic number is 19? Give reasons.
Which amongst the following radicals have valency?
