NCERT Solutions for Chapter: Metals and Non-metals, Exercise 1: Exercise
NCERT Science Solutions for Exercise - NCERT Solutions for Chapter: Metals and Non-metals, Exercise 1: Exercise
Attempt the practice questions on Chapter 3: Metals and Non-metals, Exercise 1: Exercise with hints and solutions to strengthen your understanding. Science Textbook of Competency Based Questions for Class X solutions are prepared by Experienced Embibe Experts.
Questions from NCERT Solutions for Chapter: Metals and Non-metals, Exercise 1: Exercise with Hints & Solutions
No reaction takes place when a copper plate is immersed in an aqueous solution of zinc sulphate.Explain the reason behind this.
What makes gold exist in free state in nature?
What is in the reaction?
The chart shows the process of extraction of some metals.
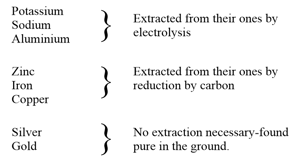 .
.
Which of these metals requires electricity for extraction from its ore?
The table shows four different materials and their resistivity.
| Magnesium | Sulphur | |
| Number of electrons in the shells of the atom |
How many atoms of sulphur will react with one atom of magnesium to form a compound?
A metal oxide on being heated with carbon does NOT produce carbon dioxide. Give a possible explanation for this behaviour of the metal oxide.
When is burnt in the atmosphere of an element X, a yellow powder is obtained. When this is dissolved in water, it gives a compound Y with a pungent smell. What are X and Y?
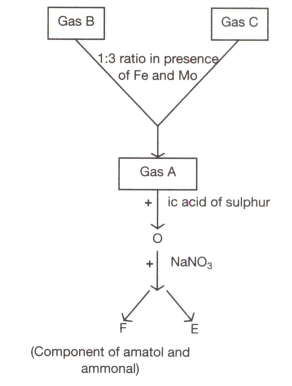
(i) Identify A, B, C, D, E and F.
(ii) Write the reactions associated with the above process and identify the types of reactions involved.
