Exercise
NCERT Science Solutions for Exercise
Simple step-by-step solutions to Exercise questions of Carbon and its Compounds from Science Textbook of Competency Based Questions for Class X. Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Exercise with Hints & Solutions
The pH of three solutions is given in the table. Answer the questions that follow.
| Solution | pH |
| P | |
| Q | |
| R |
Which of these solutions could possibly react with zinc metal to produce hydrogen gas?
A remarkable property of acids is that they can 'dissolve' metals. When metals are added to an acid, they disintegrate and disappear into the acid,
State one other common observation when metals 'dissolve' in acids, Explain the reason for this observation.
A remarkable property of acids is that they can 'dissolve' metals. When metals are added to an acid, they disintegrate and disappear into the acid.
If the acid with the 'dissolved' metal is evaporated, can we get the metal back? Why or why not?
A remarkable property of acids is that they can 'dissolve' metals. When metals are added to an acid, they disintegrate and disappear into the acid.
In this question, the word 'dissolve' is used within quotes. This is because it is not actually an example of dissolving. What is the main difference between a metal 'dissolving' in an acid and sugar dissolving in water?
Two salts X and Y are taken in two test tubes A and B, respectively and subjected to heating. Water is added to two test tubes, in case of A salt regains its original colour and in case of B water starts boiling. Then, X and Y may be, respectively:
Heating an alcohol with concentrated sulphuric acid results in the dehydration of the alcohol to give the alkene as shown by the reaction of ethanol to give ethene.
Pramila heated -butanol (shown below) with concentrated sulphuric acid.
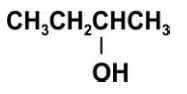
Write the structural formulae of all the possible products of the reaction.
The picture shows the bonds between atoms in two allotropes of carbon.
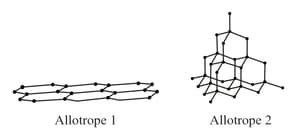
Which allotrope is harder? Explain your answer.
Dipti has three flasks containing dilute hydrochloric acid, dilute sulphuric acid and dilute sodium hydroxide respectively. The flasks are not labelled, and she does not have any pH indicator.
(a) Which of the solutions will she be able to identify just by making mixtures of pairs of the substances.
(b) What observation will help her to make this identification?
