R.R. Mishra Solutions for Exercise 3: QUESTIONS ON HIGHER ORDER THINKING SKILLS (HOTS)
R.R. Mishra Chemistry Solutions for Exercise - R.R. Mishra Solutions for Exercise 3: QUESTIONS ON HIGHER ORDER THINKING SKILLS (HOTS)
Attempt the free practice questions from Exercise 3: QUESTIONS ON HIGHER ORDER THINKING SKILLS (HOTS) with hints and solutions to strengthen your understanding. Secondary Chemistry Class X solutions are prepared by Experienced Embibe Experts.
Questions from R.R. Mishra Solutions for Exercise 3: QUESTIONS ON HIGHER ORDER THINKING SKILLS (HOTS) with Hints & Solutions
Write the atomic symbols of all the possible elements using the letters of magnesium (you may use capitals or capital formed by small letters).
There are two shells in an atom. The number of electrons in the second shell is thrice the number of electrons in the first shell. Name the element.
Name two metals each with two valence electrons.
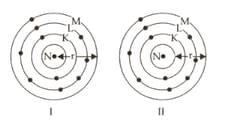
Which one of the figures I and II represent the correct measure of atomic radius (r)? Justify your answer.
Atomic symbols of first 20 elements of the periodic table are jumbled in a circle.
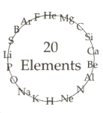
(i) Rearrange these elements in the increasing order of their atomic numbers.
(ii) Arrange these elements in their correct periods.
(iii) Arrange these elements in their correct groups.
In a period if the atomic radius of the first element is 152pm. What is the atomic radius of the third element — more than or less than 152pm?
In a group if the atomic radius, of the second element, is 152pm. What is the atomic radius of the third element — more than or less than 152pm.
What is the formula of aluminium oxide if the formula of aluminium chloride is ?
