Seema Saini Solutions for Chapter: Chemical Equilibrium, Exercise 1: DPP 6.1
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: Chemical Equilibrium, Exercise 1: DPP 6.1
Attempt the free practice questions on Chapter 6: Chemical Equilibrium, Exercise 1: DPP 6.1 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part 1 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: Chemical Equilibrium, Exercise 1: DPP 6.1 with Hints & Solutions
For the reaction , variation of concentration is plotted against time. The time at which the equilibrium establishes is as shown:
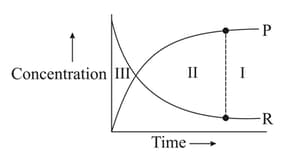
Which of the following regions show(s) equilibrium?
An aquatic species needs at least of for their survival. The solubility of in the water at and pressure is . The partial pressure of above water (in the atmosphere at ) needed for the survival of species is:
In a reversible reaction , the initial concentration of and are and , respectively, in moles per litre and the equilibrium concentration are and , respectively. Express in terms of and .
At a particular temperature and atmospheric pressure, the solid and liquid phases of a pure substance can exist in equilibrium. Which of the following term defines the temperature?
Select the correct statements.
Which are heterogeneous system?
Select the correct statements:
Which of the following sketches may represent the above equilibrium? Assume equilibrium can be achieved from either side and by taking anyone or more components initially.(Given for the reaction ).
