Seema Saini Solutions for Chapter: Ionic Equilibrium, Exercise 6: DPP 7.6
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: Ionic Equilibrium, Exercise 6: DPP 7.6
Attempt the free practice questions on Chapter 7: Ionic Equilibrium, Exercise 6: DPP 7.6 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part 1 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: Ionic Equilibrium, Exercise 6: DPP 7.6 with Hints & Solutions
An acid type indicator. differs in colour from its conjugate base The human eye is sensitive to colour differences only when the ratio is greater than or smaller than . What should be the minimum change in the of the solution to observe a colour change ?
If at , range of phenolphthalein is , then at , what would be the range of phenolphthalein?
A certain indicator (an organic dye) has . For which of the following titrations it may it be suitable?
An acid-base indicator, which is a weak acid and has a value . At what concentration ratio of sodium acetate to acetic acid would the indicator show a color halfway between those of its acid and conjugate base forms? [ of acetic acid ]
of a weak monobasic acid requires for complete titration. If the of the solution upon addition of of this alkali to of the above solution of is , then the of the weak acid is:
What is the difference in for and stages of neutralization of with ?
The pink colour of phenolphthalein in alkaline medium is due to:
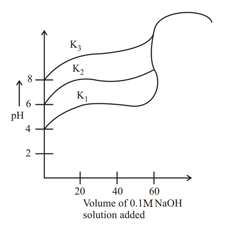
Titration curves for solutions of three weak acids and with ionization constants and , respectively as shown in figure below. The volume of added is in