Seema Saini Solutions for Chapter: States of Matter, Exercise 1: DPP 4.1
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: States of Matter, Exercise 1: DPP 4.1
Attempt the free practice questions on Chapter 4: States of Matter, Exercise 1: DPP 4.1 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part 1 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: States of Matter, Exercise 1: DPP 4.1 with Hints & Solutions
An iron cylinder contains helium at a pressure of at . The cylinder can withstand a pressure of . The room in which cylinder is placed catches fire. Predict whether the cylinder will blow up before it melts or not. Given, that the melting point of cylinder.
The ideal gas equation for of an ideal gas is .
The graph between and at constant volume, i.e., isochors are plotted as shown:
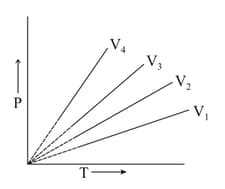
Which of the following orders of the volume is correct?
Which of the following graphs represents vs for an ideal gas at constant and ?
For a fixed mass of a gas at constant pressure, which of the following statements is/are not correct?
Boyle's law cannot be expressed as
Which of the following plots is/are correct?
Which of the following curve does not represent the Boyle's law?
Which of the following plots is/are correct?
