Seema Sinha Solutions for Chapter: Reduction and Oxidation Reactions of Organic Compounds, Exercise 2: DPP 2.2
Seema Sinha Chemistry Solutions for Exercise - Seema Sinha Solutions for Chapter: Reduction and Oxidation Reactions of Organic Compounds, Exercise 2: DPP 2.2
Attempt the practice questions on Chapter 2: Reduction and Oxidation Reactions of Organic Compounds, Exercise 2: DPP 2.2 with hints and solutions to strengthen your understanding. Daily Practice Problems (DPP) Organic Chemistry Part 2 JEE Main and Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Sinha Solutions for Chapter: Reduction and Oxidation Reactions of Organic Compounds, Exercise 2: DPP 2.2 with Hints & Solutions
An unknown hydrocarbon , with formula , react with molar equivalent of over a palladium catalyst. Hydrocarbon also react with to give a diol . When oxidized with in acidic solution, '' gives two fragments. One fragment is propanoic acid and other fragment is a ketone . What are the structures of and ?
The given reaction,
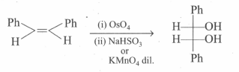
is an example of:
Product obtained in the above reaction is
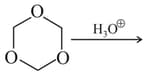
The reaction,
An organic compound on reaction with followed by and gives . The structures is/are:
Which of the following will give syn addition?
On oxidation, an unknown alkene gives a mixture of propanoic acid and pentanoic acid. The unknown alkene can be:
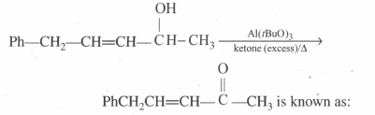
Propanol and Propanol can be best distinguished by:
