Sergey Bylikin, Gary Horner and, Brian Murphy Solutions for Chapter: Energetics and Thermochemistry, Exercise 6: Questions
Sergey Bylikin Chemistry Solutions for Exercise - Sergey Bylikin, Gary Horner and, Brian Murphy Solutions for Chapter: Energetics and Thermochemistry, Exercise 6: Questions
Attempt the practice questions on Chapter 5: Energetics and Thermochemistry, Exercise 6: Questions with hints and solutions to strengthen your understanding. Oxford IB Diploma Programme Chemistry Course Companion solutions are prepared by Experienced Embibe Experts.
Questions from Sergey Bylikin, Gary Horner and, Brian Murphy Solutions for Chapter: Energetics and Thermochemistry, Exercise 6: Questions with Hints & Solutions
But--ene gas burns in oxygen to produce carbon dioxide and water vapour according to the following equation:
The value of for the combustion of But--ene is calculated using the given data table, and is found to be . State and explain whether the given reaction is endothermic or exothermic?
| Bond | ||||||
| Average bond enthalpy () |
Given the following data:
, and . Calculate te average bond enthalpy (in ) for the bond.
Methanol is made in large quantities as it is used in the production of polymers and in fuels. The enthalpy of combustion of methanol can be determined theoretically or experimentally .
Using the data from the given table, and determine the theoretical enthalpy of combustion of methanol.
| Bond | ||||||
| Average bond enthalpy () |
The enthalpy of combustion of methanol can also be determined experimentally in a school laboratory. A burner containing methanol was weighed and used to heat water in a test tube as illustrated in figure.
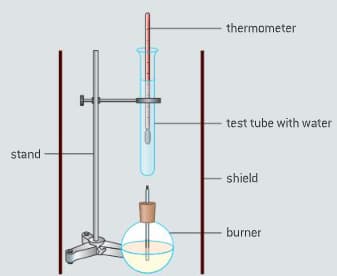
The data shown in table were collected.
| Initial mass of burner and methanol / | |
| Final mass of burner and methanol / | |
| Mass of water in test tube / | |
| Initial temperature of water / | |
| Final temperature of water |
The enthalpy of combustion of methanol can also be determined experimentally in a school laboratory. A burner containing methanol was weighed and used to heat water in a test tube as illustrated in figure.
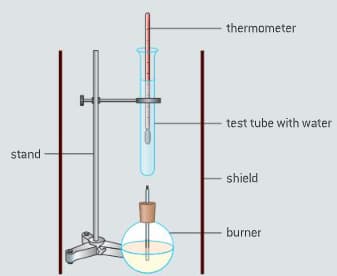
The data shown in table were collected.
| Initial mass of burner and methanol / | |
| Final mass of burner and methanol / | |
| Mass of water in test tube / | |
| Initial temperature of water / | |
| Final temperature of water |
The enthalpy of combustion of methanol can also be determined experimentally in a school laboratory. A burner containing methanol was weighed and used to heat water in a test tube as illustrated in figure.
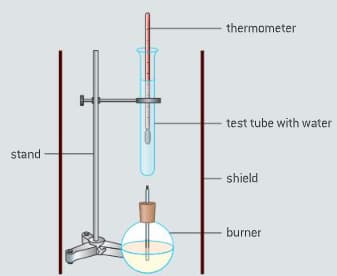
The data shown in table were collected.
| Initial mass of burner and methanol / | |
| Final mass of burner and methanol / | |
| Mass of water in test tube / | |
| Initial temperature of water / | |
| Final temperature of water |
Determine the enthalpy change, in , for the combustion of one mole of methanol.
Methanol is made in large quantities as it is used in the production of polymers and in fuels. The enthalpy of combustion of methanol can be determined theoretically or experimentally .
Using the data from the given table, the value of enthalpy of combustion of methanol is found to be.
| Bond | ||||||
| Average bond enthalpy () |
The data booklet value for the enthalpy of combustion of methanol is . Suggest why this value differs from the values calculated theoretically.
The enthalpy of combustion of methanol can also be determined experimentally in a school laboratory. A burner containing methanol was weighed and used to heat water in a test tube as illustrated in figure.
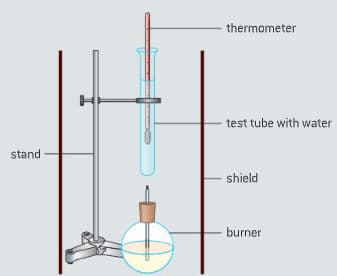
The data shown in table were collected.
| Initial mass of burner and methanol / | |
| Final mass of burner and methanol / | |
| Mass of water in test tube / | |
| Initial temperature of water / | |
| Final temperature of water |
The data booklet value for the enthalpy of combustion of methanol is . Suggest why this value differs from the values calculated experimentally.
