Shikha Goel and Saleha Parvez Solutions for Chapter: The Periodic Table, Exercise 5: EXAM PRACTICE
Shikha Goel Chemistry Solutions for Exercise - Shikha Goel and Saleha Parvez Solutions for Chapter: The Periodic Table, Exercise 5: EXAM PRACTICE
Attempt the practice questions on Chapter 5: The Periodic Table, Exercise 5: EXAM PRACTICE with hints and solutions to strengthen your understanding. All In One ICSE Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Shikha Goel and Saleha Parvez Solutions for Chapter: The Periodic Table, Exercise 5: EXAM PRACTICE with Hints & Solutions
Which group of elements could be placed in Mendeleev's periodic table without disturbing the original order? Give reason.
Mendeleev predicted the existence of certain elements not known at that time and named two of them as eka-silicon and eka-aluminium.
How many valence electrons are present in each one of them?
A part of the periodic table has been shown below: Answer the following questions on the basis of position of elements in the given table.
Which element is a noble gas? Give reason.
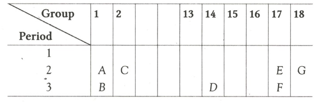
A part of the periodic table has been shown below: Answer the following questions on the basis of position of elements in the given table.
Which element is most electronegative? Give reason.
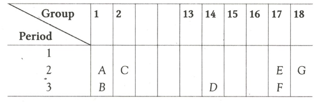
A part of the periodic table has been shown below:
Answer the following questions on the basis of position of elements in the given table.
(i) Which element is a noble gas? Give reason.
(ii) Which element is more electronegative? Give reason.
(iii) Write the electronic configuration of (a) B and (b) E.
| Group/ Period | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 | |||
| 1 | |||||||||||
| 2 | A | C | E | G | |||||||
| 3 | B | D | F |
The position of three elements A, B and C in the periodic table are shown below.
| Group 16 | Group 17 |
| -- | -- |
| -- | A |
| -- | -- |
| B | C |
(i) State whether A is a metal or non-metal.
(ii) State whether C is more reactive or less reactive than A.
(iii) Will C be larger or smaller in size than B.
(iv) Which type of ion, cation or anion, will be formed by A.
Properties of the elements are given below.
Where would you locate the following elements in the periodic table?
(i) A soft metal stored in kerosene.
(ii) An element with variable (more than one) valency stored in water.
(iii) An element which is tetravalent and forms the basis of organic chemistry.
(iv) An element which is an inert gas with atomic number 2.
(v) An element whose thin oxide layer is used to make other elements corrosion resistant by the process of 'anodising'.
Atomic number of a few elements are given below.
(i) Identify the elements.
(ii) Identify the group number of these elements in the periodic table.
(iii) Identify the periods of these elements in the periodic table.
(iv) What would be the electronic configuration for each of these elements.
(v)Determine the valency of these elements.
