Sourabh Monga Solutions for Chapter: Carbon and Its Compounds, Exercise 6: SELF-EVALUATION TEST
Sourabh Monga Chemistry Solutions for Exercise - Sourabh Monga Solutions for Chapter: Carbon and Its Compounds, Exercise 6: SELF-EVALUATION TEST
Attempt the free practice questions on Chapter 4: Carbon and Its Compounds, Exercise 6: SELF-EVALUATION TEST with hints and solutions to strengthen your understanding. New Millenium Science - Chemistry Class X solutions are prepared by Experienced Embibe Experts.
Questions from Sourabh Monga Solutions for Chapter: Carbon and Its Compounds, Exercise 6: SELF-EVALUATION TEST with Hints & Solutions
Name the following compound:
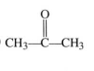
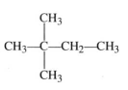
Name the following compound:
Atomic numbers of three elements X, Y and Z are , and respectively.
(a) Write down the molecular formula of the compound formed by elements ‘X’ and ‘Y’.
(b) Explain the bonding of other compounds formed.
Draw the possible isomers of the compound with molecular formula and also give their electron dot structures.
A neutral organic compound X with molecular formula , on oxidation with potassium dichromate and sulphuric acid gives an acidic compound Y. The compound X on warming with Y in presence of concentrated sulphuric acid gives a sweet smelling substance Z. Identify X, Y and Z.
Soaps and detergents are both type of salts. State the difference between the two. Write the mechanism of the cleansing action of soaps. Why do soaps not form lather (foam) with hard water? Mention any two problems that arise due to use of detergents instead of soaps.
Identify the compounds , and in the following reaction sequence :
(a)
(b)
(c)
What is glacial acetic acid? Why is it named so? State its two uses.
