Olympiad/HOTS corner
Subject Experts Chemistry Solutions for Exercise - Olympiad/HOTS corner
Simple step-by-step solutions to Olympiad/HOTS corner questions of Chemical Reactions And Equations from Foundation Course Chemistry. Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Olympiad/HOTS corner with Hints & Solutions
In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
The equation represents
Decomposition reaction
Displacement reaction
Combination reaction
Double displacement reaction
Redox reaction
The energy diagram shows the energy levels of reactants and products of a particular reaction.
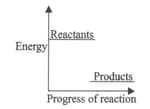
Which of the following processes can be related to the given diagram?
The chemical reactions and their corresponding observable features are matched below. The correct option is:
| Chemical reactions | Observable features | ||
| Change in temperature | Magnesium reacting with dilute sulphuric acid | ||
| Evolution of gas | Potassium iodide with lead nitrate | ||
| Formation of precipitate | Sulphur dioxide gas reacting with acidified potassium dichromate solution | ||
| Change in colour | Zinc granules reacting with dilute sulphuric acid |
A science teacher wrote the following statements about rancidity:
When fats and oils are reduced, they become rancid.
In chips packet, rancidity is prevented by oxygen.
Rancidity is prevented by adding antioxidants.
Select the correct option.
Which on of the following is an example of a redox reaction?
Classify each of the following reactions:
and are three metals that undergo chemical reactions as follows:
Observe the reactions and arrange the metals in the increasing order of their reactivity.
