Tamil Nadu Board Solutions for Chapter: Ionic Equilibrium, Exercise 3: EVALUATION
Author:Tamil Nadu Board
Tamil Nadu Board Chemistry Solutions for Exercise - Tamil Nadu Board Solutions for Chapter: Ionic Equilibrium, Exercise 3: EVALUATION
Attempt the free practice questions on Chapter 1: Ionic Equilibrium, Exercise 3: EVALUATION with hints and solutions to strengthen your understanding. Chemistry Standard 12 Vol II solutions are prepared by Experienced Embibe Experts.
Questions from Tamil Nadu Board Solutions for Chapter: Ionic Equilibrium, Exercise 3: EVALUATION with Hints & Solutions
HARD
12th Tamil Nadu Board
IMPORTANT
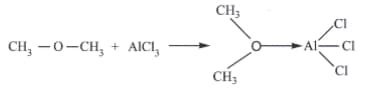
Identify the Lewis acid and the Lewis base in the following reactions.
HARD
12th Tamil Nadu Board
IMPORTANT
Identify the Lewis acid and the Lewis base in the following reactions.
MEDIUM
12th Tamil Nadu Board
IMPORTANT
of is . Calculate molar solubility in .
HARD
12th Tamil Nadu Board
IMPORTANT
A particular saturated solution of silver chromate has . What is the value of for ?
HARD
12th Tamil Nadu Board
IMPORTANT
Write the expression for the solubility product of
HARD
12th Tamil Nadu Board
IMPORTANT
of is . What is solubility of in ?
HARD
12th Tamil Nadu Board
IMPORTANT
Will a precipitate be formed when of and of are mixed?
HARD
12th Tamil Nadu Board
IMPORTANT
of . At what does precipitate on the addition of buffer of and solution?
