V. K. Sally and R. Goel Solutions for Chapter: Acids, Bases And Salts, Exercise 3: EXERCISE 2C
V. K. Sally Chemistry Solutions for Exercise - V. K. Sally and R. Goel Solutions for Chapter: Acids, Bases And Salts, Exercise 3: EXERCISE 2C
Attempt the practice questions on Chapter 2: Acids, Bases And Salts, Exercise 3: EXERCISE 2C with hints and solutions to strengthen your understanding. Core Science Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from V. K. Sally and R. Goel Solutions for Chapter: Acids, Bases And Salts, Exercise 3: EXERCISE 2C with Hints & Solutions
Which one of the following is not a neutral salt?
Plaster of Paris on mixing with water forms fine crystals of:
Match the chemical substances given in Column (A) with their appropriate application given in Column (B).
| Column(A) | Column (B) | ||
| (a) | Bleaching powder | (i) | Preparation of glass |
| (b) | Baking soda | (ii) | Production of H2 and Cl2 |
| (c) | Washing soda | (iii) | Decolourisation |
| (d) | Sodium chloride | (iv) | Antacid |
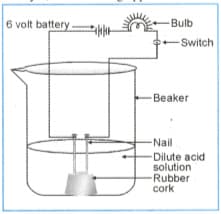
In an attempt to demonstrate electrical conductivity through an electrolyte, the following apparatus was set up. Which among the following statement(s) is (are) correct?
(i) Bulb will not glow because electrolyte is not acidic
(ii) Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is incomplete
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
Identify the correct representation of reaction occurring during Chloralkali process.
For making cake, baking powder is taken. If at home your mother uses baking soda instead of baking powder in cake. How can baking soda be converted into baking powder?
For making cake, baking powder is taken. If at home your mother uses baking soda instead of baking powder in cake. What is the role of tartaric acid added to baking soda?
A metal carbonate X on reacting with an acid gives a gas which when passed through a solution Y gives the carbonate back. On the other hand, a gas G that is obtained at the anode during electrolysis of brine when passed on dry Y, gives a compound Z, used for disinfecting drinking water. Identify X, Y, G and Z.
