West Bengal Board Solutions for Chapter: Occurence of Carbon and its Compounds in Nature, Exercise 4: Exercise 4
West Bengal Board Science Solutions for Exercise - West Bengal Board Solutions for Chapter: Occurence of Carbon and its Compounds in Nature, Exercise 4: Exercise 4
Attempt the practice questions on Chapter 4: Occurence of Carbon and its Compounds in Nature, Exercise 4: Exercise 4 with hints and solutions to strengthen your understanding. Environment and Science Class 8 solutions are prepared by Experienced Embibe Experts.
Questions from West Bengal Board Solutions for Chapter: Occurence of Carbon and its Compounds in Nature, Exercise 4: Exercise 4 with Hints & Solutions
Reaction of metal carbonate and bicarbonate with dilute acid to produce gas.
Reaction of metal carbonate and bicarbonate with dilute acid to produce gas.
Complete the reaction of metal carbonate and bicarbonate with dilute acid to produce gas given below:
.
Reaction of metal carbonate and bicarbonate with dilute acid to produce gas.
..
and are insoluble in water. Is it possible to get gas by the reaction of with dilute or ?
Production of gas by heating metal carbonates or bicarbonates
Production of gas by heating metal carbonates or bicarbonates
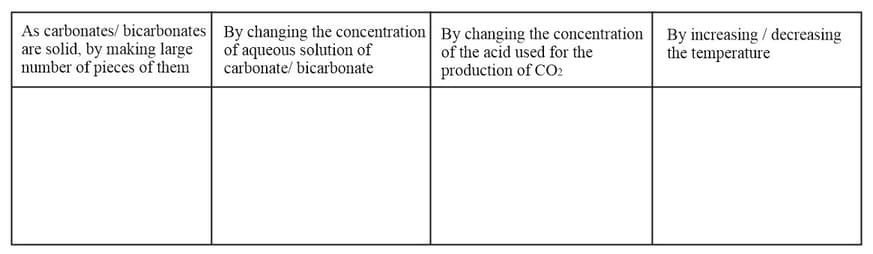
How can the following conditions influence the production of carbon dioxide?